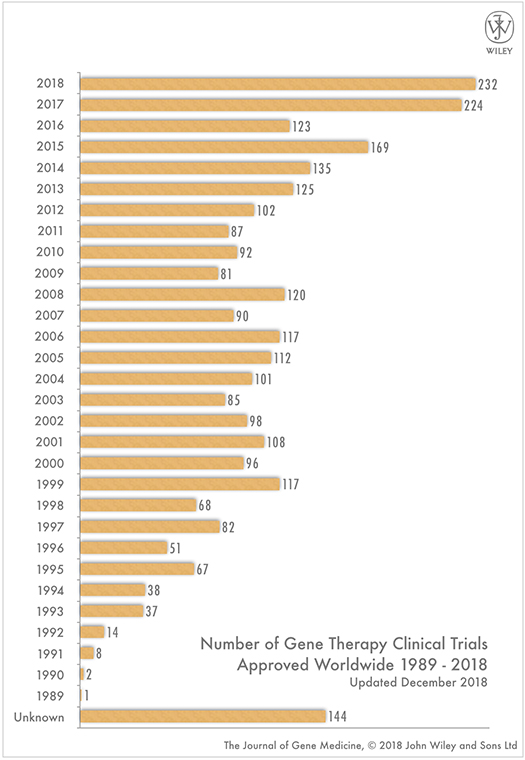
 Human gene therapy is a rapidly growing area, as evidenced by the growing number of clinical trials initiated where an Institutional Biosafety Committee (IBC) or Genetically Modified Organism (GMO) risk assessment review is required. IBC review and oversight are a critical component for reducing risks to study staff, research participants, and the environment involving genetically modified biological materials. Sponsors, institutions and sites that have either received NIH funds or are conducting NIH-funded studies involving gene therapy are required by the United States NIH Guidelines to have IBC oversight.
Human gene therapy is a rapidly growing area, as evidenced by the growing number of clinical trials initiated where an Institutional Biosafety Committee (IBC) or Genetically Modified Organism (GMO) risk assessment review is required. IBC review and oversight are a critical component for reducing risks to study staff, research participants, and the environment involving genetically modified biological materials. Sponsors, institutions and sites that have either received NIH funds or are conducting NIH-funded studies involving gene therapy are required by the United States NIH Guidelines to have IBC oversight.
CBS Provides Turn-Key IBC Review Services:
Ready to submit a project for a CBS risk assessment review?